August 4th, 2021 | 11:10 CEST
XPhyto Therapeutics - Groundbreaking diagnostic platforms of the future
The number of infectious diseases that we humans are fighting today has increased significantly in recent decades, not just because they have become known and researched. Increasingly frequent variants and neoplasms have also led in turn to entirely new disease patterns. An important area upstream of the medical sector is the large diagnostics market. The umbrella term of diagnostics is used in particular in the context of the detection and differentiation of diseases. This is referred to as clinical diagnostics. Many test and diagnostic procedures are assigned to the bioscience sector since studies must first prove their exact validity. Worldwide, more than 10,000 companies are involved in symptom research, the detection, screening and analysis of the diagnosis of diseases.
time to read: 12 minutes
|
Author:
André Will-Laudien
ISIN:
XPHYTO THERAPEUTICS | CA98421R1055
Table of contents:

"[...] As a company dedicated to developing treatments for rare heart diseases, we see this as an opportune moment to contribute to the fight against heart disease and make meaningful strides in improving heart health worldwide. [...]" David Elsley, CEO, Cardiol Therapeutics Inc.
Author
André Will-Laudien
Born in Munich, he first studied economics and graduated in business administration at the Ludwig-Maximilians-University in 1995. As he was involved with the stock market at a very early stage, he now has more than 30 years of experience in the capital markets.
Tag cloud
Shares cloud
XPhyto Therapeutics – future of diagnostic platforms
Well-positioned in this highly competitive market is life science accelerator XPhyto Therapeutics, whose focus is on investing in next-generation drug delivery, diagnostics and new active pharmaceutical ingredients. The accelerator approach finds its application primarily in the startup world and is intended to describe the business model of an accelerator. Here, the activity at a biotech company such as XPhyto corresponds to the active advancement of research approaches in the form of an incubator. Often, this is done in collaboration with universities and research institutes, which are connected in the form of collaborations and work contracts. The common purpose is the discovery and further development of diagnostic methods.
Corona pandemic as a test case for our fragile healthcare system - PCR test elementary for detection
Viral pathogens infect the human organism even more frequently than bacterial ones. Their unique form of reproduction makes it particularly difficult for the immune system to fight the infection. If viruses enter the body, the infected cells themselves are used for virus replication. As a result, the host cell produces thousands of new viruses and releases them. When the Covid-19 virus took off in 2019/2020, there were no known ways to intervene against the ongoing pandemic outside of ongoing flu vaccinations.
However, valid tests of infections are essential for the identification of the infection process. Thus, testing methods have been developed that indicate infection after the incubation period (rapid antigen test) or detect disease that has already been passed (antibody test). The health care system must admit infected people, establish meaningful isolation, and manage effective cures within a manageable time. The utilization of emergency beds and the availability of trained clinical staff were considered important alerts to the infectious situation within the population and called on governments to take strict countermeasures.
The PCR test is indispensable in diagnostics; it is now referred to as the gold standard among corona tests. It can reliably detect the presence of pathogens in a sample from the mucous membranes of the respiratory tract. The diagnostic power lies in establishing the correct threshold of how much pathogen material is actually defined as "infectious." Viral load is the amount of virus found in blood serum, blood plasma, sputum or a throat swab. The PCR test is a standard procedure in the diagnosis of viruses. The test is based on the so-called polymerase chain reaction (PCR). In this process, the virus's genetic material is amplified, and it is possible to detect viruses even if only a few pathogens are present. Therefore, the PCR test has high sensitivity - it detects the virus with a high degree of accuracy. In addition, only the genetic material of the SARS-CoV-2 coronavirus is amplified, so the test has high specificity.
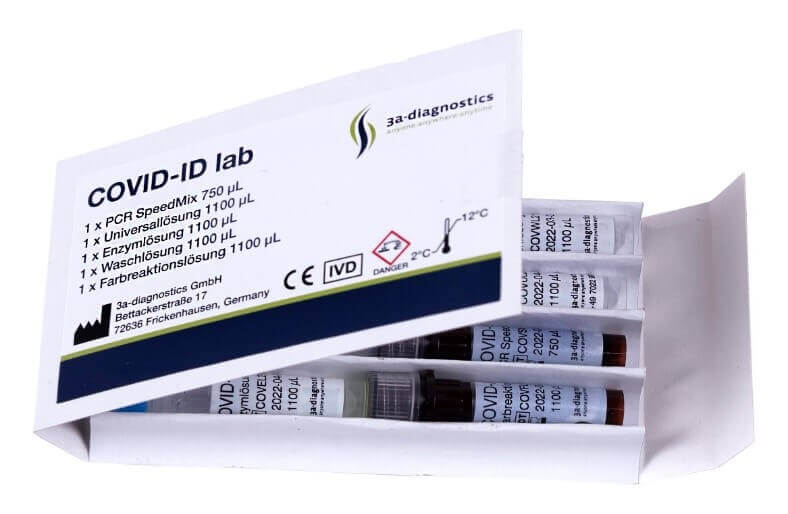
XPhyto Therapeutics has been working with 3a-diagnostics GmbH since 2020 to develop a universally applicable PCR test that enables rapid detection of infectious diseases at the point of care. Research on this was completed in early 2021, and the first test product was approved throughout the EU. Sales of the Covid-19 RT-PCR test system "Covid-ID Lab" started in Germany at the end of May. The advantage of the test offered is that Covid-ID Lab requires only a 20-minute evaluation time without prior RNA extraction as part of sample preparation. The product is intended to bridge the gap between single-use antigen tests and conventional PCR systems. Application areas are primarily locations with reduced stand-off areas and restricted access areas requiring up-to-date and rapid COVID testing, such as border crossings, airports, cruise ships, food & event venues, pharmacies and medical care facilities, as well as industrial and educational sites.
3a-diagnostics is a research-oriented biotechnology company near Stuttgart, Germany, specializing in developing, producing, and marketing point-of-care testing systems. It has developed a range of molecular biosensor screening tests for bacterial and viral infectious diseases, including stomatitis, peri-implantitis, periodontitis, group A streptococcus and influenza A. The Company has also developed a scalable next-generation microbial-enzymatic screening tool for high-throughput identification of biosensor targets to facilitate the rapid development of new tests.

XPhyto Therapeutics currently supplies 10 Berlin Covid-19 test centers with its rapid PCR test with on-site sample processing. The delivery of approximately 1,000 Covid-ID Lab tests to the test centers kicks off a short testing phase to integrate and evaluate the new PCR test system. "Our Covid-ID Lab mobile test is expected to be one of the fastest PCR systems in the world, while our platform is economical for low to medium sample volumes. We anticipate strong and continued demand for our rapid and versatile PCR system, " said Hugh Rogers, CEO and director of XPhyto Therapeutics.
On the trail of all virus variants with biosensor technology
The currently marketed PCR assay results must be considered groundbreaking, with the first saliva-activated biosensor molecules identified to diagnose Covid-19 infection using XPhyto's oral-dissolvable delivery platform. Biosensor candidates offer an innovative and cost-effective diagnostic alternative to conventional antigen and RNA assays. The full development of a biosensor screening test would expand XPhyto's diagnostic product pipeline to identify Covid-19 and other infectious diseases.
A comprehensive review of current Covid-19 detection methods from laboratory-based to point-of-care diagnosis, published July 24, 2021, in Science Direct, points to the importance of biosensing. As noted in the review article, conventional coronavirus detection techniques such as CT scan, PCR, sequencing, CRISPR, ELISA, LFA, and LAMP are insufficient to meet all testing needs. The urgent global need for rapid, accurate, and cost-effective detection and the need to study and rapidly identify current infectious diseases and future pandemic threats drive scientists to develop new technologies.
Biosensors in general and 3a's innovative biosensor system are a promising and reliable platform technology for accurate, early diagnosis and screening. They offer advantages over conventional detection methods. For XPhyto Therapeutics, years of research in the current pandemic confirm this. From a purely analytical point of view, the probable outbreak of the next wave of infections in the fall of 2021 may already prove the relevance of XPhyto's platforms.
By then, the share will have made a mighty move because if further intensive testing is required due to a low vaccination rate or permanently high incidence figures, XPhyto, together with its partner 3a-diagnostics, will be on hand.
Scaling determined by the current pandemic situation
Dr. Heinrich Jehle, CEO of 3a-diagnostics GmbH, states: "We are very pleased with the successful identification of the first biosensor candidates for the diagnosis of Covid-19. In the future, this will allow us to expand our portfolio of Covid-19 diagnostics and complement our recently launched 25-minute rapid PCR test 'Covid-ID Lab'. After optimization, we will evaluate the clinical performance of our new candidates and proceed with the commercial development of this novel screening test product. It is an important step in the development of next-generation Covid-19 assays, and we are optimistic that development will lead to new, cost-effective, rapid, reliable and easy-to-use diagnostic options for low-threshold surveillance of the ongoing pandemic."
Based on the already close collaboration, a framework agreement for the complete acquisition of 3a-diagnostics GmbH was also concluded in June 2021 and first announced by XPhyto on July 20, 2021. Under the agreement, XPhyto will acquire all outstanding shares in 3a for an immediate cash component of EUR 400,000 and an additional EUR 3.5 million payable by the closing of the transaction, which is scheduled for October 31, 2021.
Wolfgang Probst, Director Germany and Chief Operating Officer of XPhyto added: "We believed in 3a's research and development plan from the very beginning when we signed the first collaboration agreement in 2020. Now that XPhyto has announced the full acquisition of 3a, we are particularly excited about this development's success. The successful validation of the first biosensor candidates demonstrates 3a's expertise and scientific excellence. Following the jointly developed and successfully CE-marked 25-minute PCR assay in March of this year, this novel approach to oral biosensor screening is another important milestone to effectively identify Covid-19 infection outbreaks, contain the spread of the pandemic, and help find our way back to the new normal."
The next quantum leap: developing psychedelic-based therapeutics
An exclusive agreement with the University of Alberta enables XPhyto to work with Prof. Dr. Raimar Löbenberg to incorporate a range of psychedelic compounds, precursor molecules and metabolites into its research. Prof. Dr. Löbenberg is the founder and director of the Centre for Drug Development and Innovation, Faculty of Pharmacy and Pharmaceutical Sciences, University of Alberta, Edmonton, Canada. He holds "Health Canada licenses" for research and analytical testing of a broad range of psychedelic compounds under the Controlled Drugs and Substance Act and licenses for research and analytical testing under the Cannabis Act. Already since 2018, the Company has been using the commercial development rights of the cannabis licenses. Since 2021, this agreement now also includes psychedelic compounds. Selected substances include psilocybin, mescaline, LSD, MDMA and DMT. The therapeutic value is believed to be in their positive influence on the growth and reorganization of human neuronal networks. The initial focus is to develop standardized drug formulations with precise, predictable and efficient drug delivery for clinical trials and therapeutic use.

Psychedelic compounds are emerging as a new class of drugs with the potential for significant impact in treating medical indications related to mental health such as depression, anxiety, addiction, anorexia, and post-traumatic stress disorder. Psilocybin was recently designated twice by the US Food and Drug Administration as a "breakthrough therapy" to treat the major depressive disorder and treatment-resistant depression. Back in November 2020, XPhyto announced a research agreement with a leading German university for the exclusive development of a proprietary biotechnological process for the industrial production of "psilocybin as a certified active pharmaceutical ingredient" ("API").
The subsidiary Vektor Pharma TF GmbH: drug delivery systems
In addition to test kits, XPhyto Therapeutics also offers solutions to help the human body better absorb medical agents, such as patches and oral delivery systems. The drug formulation business focuses on neurological indications with significant market demand and the potential for significant patient impact. In 2020, the German subsidiary, Vektor Pharma TF GmbH, a leader in the development of transdermal and sublingual drug formulations, reported significant progress in four therapeutic development programs. Vektor also successfully developed a sublingual drug formulation on behalf of a major generic drug manufacturer and distributor.
Transdermal systems have been documented in principle since Egyptian times (ca. 2,000 BC). At that time, they consisted of envelopes impregnated with substances to provide occlusive conditions. In this way, the active ingredients were able to penetrate well through the skin. Since about 1980, transdermal systems have been experiencing a renaissance. This relatively new form of medication belongs to the group of parenteral administrations and is convincing because it is easy to apply over several days. It is therefore particularly suitable for use in geriatrics. There are a large number of active ingredients that have not yet been investigated for transdermal availability.
With current understanding, Transdermal Therapeutic Systems (TTS) are drug-containing films that can be adhered to the skin and remain on the skin for 1 to 7 days. During this time, the TTS continuously releases active ingredients through the skin into the bloodstream. Transdermal Therapeutic Systems are considered parenteral sustained-release drugs because their action occurs bypassing the gastrointestinal tract and the first-pass effect. Colloquially, these systems are also referred to as "drug patches." Examples include TTS for smoking cessation ('smoking patches') and pain reduction. Locally acting 'dermal systems' are films formulated so that the active ingredient cannot pass into the bloodstream but remains in the skin. The duration of application is 12-24 hours. These systems are considered topical dosage forms. As an example, pain patches are applied just before injections. Orally Disintegrating Films (ODF) are bioadhesive films that rapidly disintegrate on the tongue or in the mouth and release active ingredients in the mouth. This results in a faster onset of action. Areas of application include colds, vitamin substitution and emergencies.
In 2021, the Company plans to complete human studies for its four leading therapeutic products: 1. Rotigotine - transdermal patch for Parkinson's disease, 2. CBD - oral/sublingual strip for treatment-resistant epilepsy, 3. THC - oral/sublingual strip for anorexia/nausea, and 4. CBD:THC (1:1) - oral/sublingual strip for multiple sclerosis-associated spasticity.
For XPhyto Therapeutics, drug delivery and formulation research are important building blocks in the therapeutic context. The area should grow very dynamically in the coming years due to its high relevance in the context of minor unexplored diseases.
Acceleration from 0 to 100: a milestone plan the likes of which you rarely see
The Company's milestone plan reads like a perfect sequence of diagnostic achievements in various indications. In Covid-19, XPhyto has already passed all clinical testing and was able to go to market in the second quarter. The 3a-PCR test has been successfully used in the field since May 2021 and impresses with its rapid results.
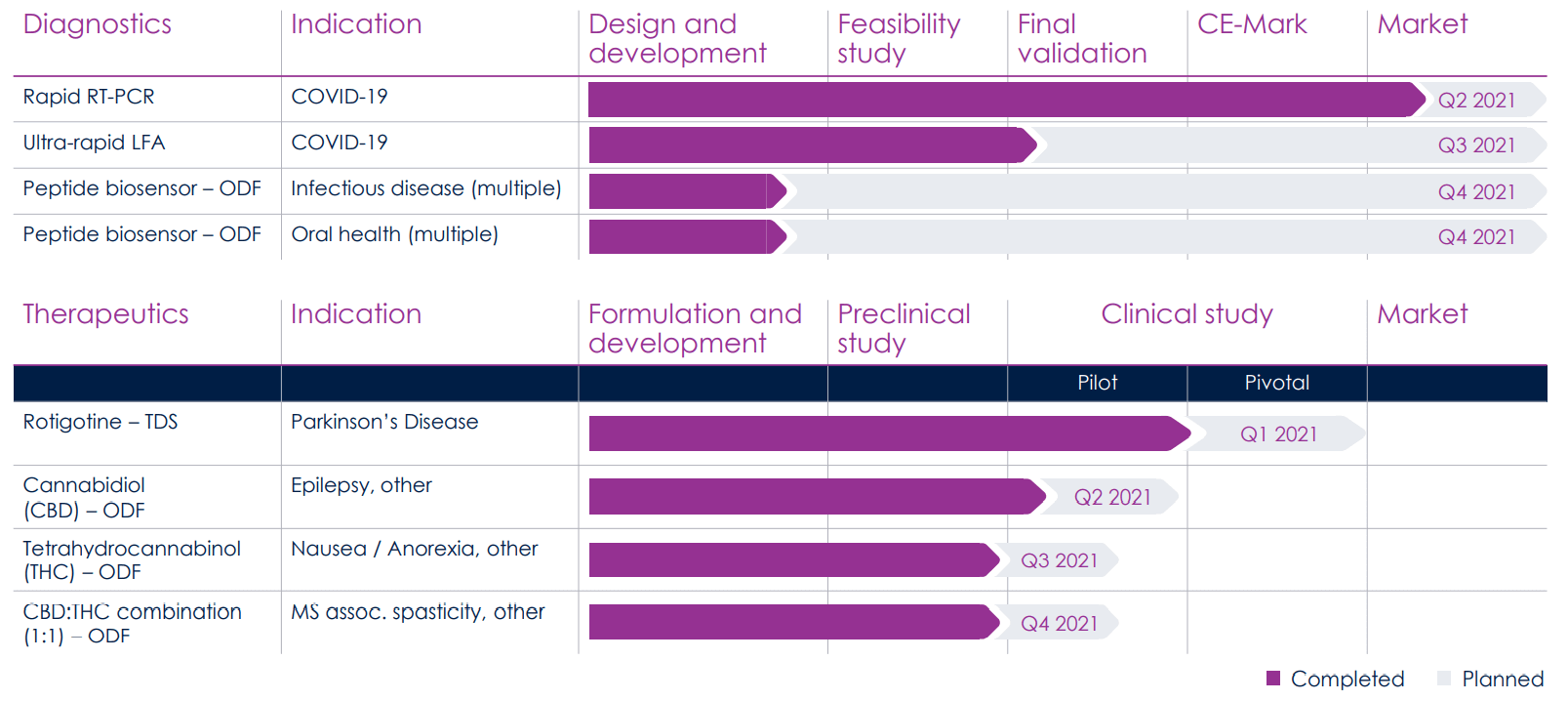
Huge potential is also seen in the area of psychedelics, with 160 clinical trials currently being conducted worldwide. XPhyto is already on the trail of the chains of action. Still, so far, it has refrained from conducting its own studies due to the high costs involved and is instead relying on the development of the active ingredient via genetically modified bacteria. In mid-June this year, the Company announced that its GMP mescaline synthesis program is right on schedule with the completion of the first production batches. The production of pharmaceutical-grade and industrial-scale psychedelic compounds, including mescaline and psilocybin, is integral to XPhyto's psychedelic medicine program. We expect some more groundbreaking developments from this line of research, as research into psilocybin applications, in particular, is still in its infancy internationally.
XPhyto Therapeutics as an investment highlight for a balanced diagnostics portfolio
XPhyto Therapeutics Corp. is a Canadian-based bioscience accelerator focused on investing in next-generation drug delivery, diagnostics and new pharmaceutical entities. The Company has research and development sites in North America and Europe, with an operational focus in Germany, and is currently focused on regulatory approval and commercialization of medical products for European markets. On the one hand, the business approach is research-oriented, but at the same time, opportunistically focused on future topics and opportunities. We assume high expertise in XPhyto Therapeutics in drug delivery, diagnostics & testing, and pending research on cannabinoids and psychedelics. Due to the future-oriented focus, new topics and research fields are constantly coming up, which, as in the case of 3a-diagnostics, also quickly result in an M&A action. The group will continue to grow under a strong German focus and enter into a number of significant collaborations.
With 69.3 million shares, XPhyto Therapeutics is valued at just EUR 88.7 million at a current price of EUR 1.28. Research-oriented companies within the biotech sector are often valued at a multiple if they can come up with human medical solutions, as in the case of XPhyto Therapeutics. Therefore, in addition to key study results, upcoming commercial successes from the product pipeline for XPhyto Therapeutics should very quickly lead to a dramatic upside in value, in our view. As the Company is very opportunistic, suitable external portfolio additions can also create significant value.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may in the future hold shares or other financial instruments of the mentioned companies or will bet on rising or falling on rising or falling prices and therefore a conflict of interest may arise in the future. conflict of interest may arise in the future. The Relevant Persons reserve the shares or other financial instruments of the company at any time (hereinafter referred to as the company at any time (hereinafter referred to as a "Transaction"). "Transaction"). Transactions may under certain circumstances influence the respective price of the shares or other financial instruments of the of the Company.
Furthermore, Apaton Finance GmbH reserves the right to enter into future relationships with the company or with third parties in relation to reports on the company. with regard to reports on the company, which are published within the scope of the Apaton Finance GmbH as well as in the social media, on partner sites or in e-mails, on partner sites or in e-mails. The above references to existing conflicts of interest apply apply to all types and forms of publication used by Apaton Finance GmbH uses for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and etc. on news.financial. These contents serve information for readers and does not constitute a call to action or recommendations, neither explicitly nor implicitly. implicitly, they are to be understood as an assurance of possible price be understood. The contents do not replace individual professional investment advice and do not constitute an offer to sell the share(s) offer to sell the share(s) or other financial instrument(s) in question, nor is it an nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but rather financial analysis, but rather journalistic or advertising texts. Readers or users who make investment decisions or carry out transactions on the basis decisions or transactions on the basis of the information provided here act completely at their own risk. There is no contractual relationship between between Apaton Finance GmbH and its readers or the users of its offers. users of its offers, as our information only refers to the company and not to the company, but not to the investment decision of the reader or user. or user.
The acquisition of financial instruments entails high risks that can lead to the total loss of the capital invested. The information published by Apaton Finance GmbH and its authors are based on careful research on careful research, nevertheless no liability for financial losses financial losses or a content guarantee for topicality, correctness, adequacy and completeness of the contents offered here. contents offered here. Please also note our Terms of use.




