November 18th, 2025 | 07:05 CET
Patents, potential, takeover candidate? Making money with less risk: The secret of BioNxt Solutions
In the high-risk world of biotechnology, the path from the laboratory to an approved drug is often an ordeal. Canadian company BioNxt Solutions may have found the key to a less painful path. Instead of developing expensive and uncertain new active ingredients, the Company refines proven drugs with innovative administration technology. The past few months have brought a remarkable series of successes that not only confirm the technology but also significantly increase the strategic value of the Company. For investors, this could be a rare opportunity.
time to read: 4 minutes
|
Author:
Armin Schulz
ISIN:
Bionxt Solutions Inc. | CA0909741062
Table of contents:

"[...] As a company dedicated to developing treatments for rare heart diseases, we see this as an opportune moment to contribute to the fight against heart disease and make meaningful strides in improving heart health worldwide. [...]" David Elsley, CEO, Cardiol Therapeutics Inc.
Author
Armin Schulz
Born in Mönchengladbach, he studied business administration in the Netherlands. In the course of his studies he came into contact with the stock exchange for the first time. He has more than 25 years of experience in stock market business.
Tag cloud
Shares cloud
Playing with less risk: Making proven products better
Those who invest in biotech get used to setbacks. Clinical trials fail, approvals are delayed, and billions are burned. BioNxt Solutions pursues an approach that avoids many of these pitfalls from the outset. Its core business is not the invention of new active ingredients, but their optimization through advanced delivery systems. Specifically, these are sublingual thin films that dissolve under the tongue.
Why is this a game-changer? The active ingredients BioNxt works with are already approved, and their safety and efficacy have been proven. The Company does not have to spend years conducting costly, large-scale studies to prove that its product works in principle. Instead, it is focusing on demonstrating bioequivalence, proving that the active ingredient in its own dissolvable film is absorbed by the body just as well as in the original tablet or injection. This process is significantly faster, more cost-effective, and more predictable.
October and November: A series of strategic successes
The past few weeks have provided impressive evidence of the viability of this model:
• Strong patent protection: In early November, BioNxt received a "notice of intent to grant a patent" from the European Patent Office for its sublingual cladribine formulation (BNT23001) for the treatment of multiple sclerosis (MS). This followed a similar positive announcement from the Eurasian Patent Organization in late October. Taken together, BioNxt has secured potential protection in up to 54 countries, a massive step for a company of its size. This patent shield is not a paper tiger; it protects the specific composition of the film containing a cladribine-cyclodextrin complex, making imitation unattractive for years to come.
• Progress in the laboratory: On October 21, BioNxt launched a comprehensive bioequivalence study in animals with higher body weights. This study is the final animal step in precisely determining the dosage for the pivotal human study to begin in early 2026. The results are expected in December, a clear short-term catalyst. CEO Hugh Rogers emphasized: "This study in animals with higher body weight should enable a more precise formula for our human study...". If the data confirms the high bioavailability of the film, this would be a strong signal for future partners.
• Turbo for the US market: Not to be forgotten is the Track One priority application to the US Patent Office on October 9. This "fast-track" procedure promises a decision within just 9-12 months and shows how aggressively BioNxt is approaching its most valuable market.
The flagship: A piece of film for a billion-dollar market
At the center of the hype is BNT23001, a dissolvable film containing the active ingredient cladribine. This drug is already on the market as a tablet (Merck's Mavenclad) and has proven itself in the treatment of relapsing multiple sclerosis. The market is enormous. In Europe alone, the MS therapy volume is estimated at over USD 13 billion by 2032.
So why should a patient switch from the proven tablet to the BioNxt film? The advantages are tangible:
-
No swallowing required: An enormous advantage for MS patients, who often suffer from difficulty swallowing (dysphagia).
-
Discreet and convenient: It can be taken without water and in seconds, which promotes therapy adherence.
-
Potentially better drug absorption: Absorption through the oral mucosa could bypass the "first-pass effect" of the liver, enabling higher bioavailability. More active ingredient at the target site with a potentially lower dose is the holy grail of drug delivery.
"Securing patent protection in both Europe and Eurasia is an important step forward in our global strategy for intellectual property protection and commercialization," said Rogers. These patents significantly strengthen the negotiating position.
The business model: Development, not marketing
BioNxt plans to license the developed products to large pharmaceutical companies after successful approval. It may also become a takeover candidate. For a pharmaceutical giant looking to expand its portfolio or protect its own product from imitators, a reformulated, patient-friendly drug with strong patent protection is an extremely attractive asset. The targeted annual revenues of over USD 100 million within five years would be entirely realistic with such licensing agreements.
Looking ahead: More than just multiple sclerosis
While BNT23001 is the pioneer, the platform is not limited to this one active ingredient or indication. The technology has a wide range of applications. BioNxt has programs in the pipeline for myasthenia gravis, lupus nephritis, and even longevity with active ingredients such as everolimus. Even more visionary, but no less promising, is a completely separate oncology platform for targeted chemotherapy, which is designed to release toxic agents precisely in the tumor. The research team is already working intensively to identify the first candidate.
Analysts at Black Research see great potential in the stock and have set a price target of EUR 2.50. The stock is currently trading at just CAD 0.77, which corresponds to around EUR 0.47.
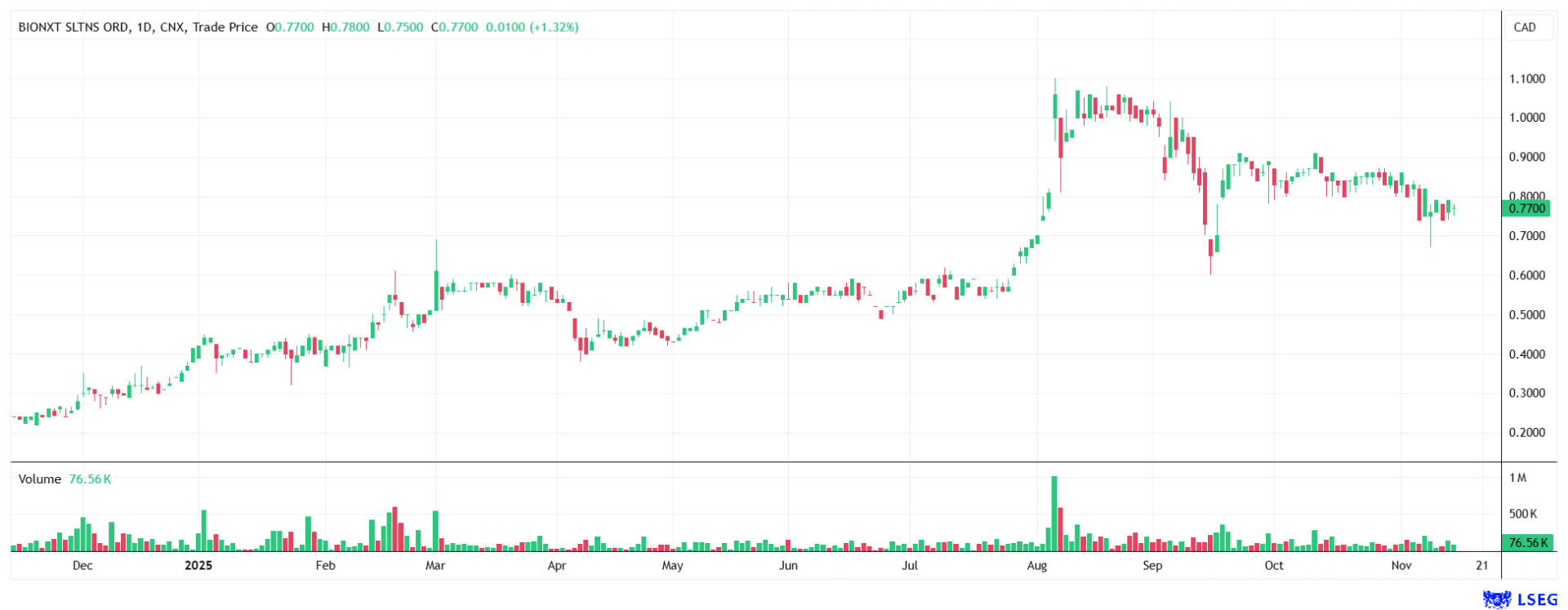
BioNxt Solutions offers a package that is rare in the biotech world, with disruptive technology based on a low-risk and capital-efficient development pathway. Recent successes in securing global patents and rapid progress in preclinical development underscore the excellence of the approach and the execution strength of the team. For investors who want to participate in the fundamental growth drivers in healthcare without taking on the extreme risks of traditional biotech bets, this Company represents a compelling and timely opportunity. The coming weeks, with the study results, could be the catalyst for a new valuation level.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may hold shares or other financial instruments of the aforementioned companies in the future or may bet on rising or falling prices and thus a conflict of interest may arise in the future. The Relevant Persons reserve the right to buy or sell shares or other financial instruments of the Company at any time (hereinafter each a "Transaction"). Transactions may, under certain circumstances, influence the respective price of the shares or other financial instruments of the Company.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.




