March 6th, 2025 | 07:00 CET
NETRAMARK HOLDINGS – Medical Revolution: How AI is Shortening Drug Approval by Years!
Artificial Intelligence – now it is really taking off in the medical field! Among many other applications, big data and artificial intelligence (AI) applications are revolutionizing the optimization of clinical phases in drug approval by accelerating processes, reducing costs, and significantly increasing the success rate. AI can analyze large amounts of data from electronic health records, genomic data, and patient registries to identify suitable clinical trial participants more quickly and accurately. This significantly reduces recruitment time and improves accuracy. Machine learning can be used to analyze previous study results to develop more efficient study designs. Based on real-time data, adaptive study protocols enable dynamic adjustment of experimental conditions. The Canadian technology company NetraMark Holdings Inc. (WKN: A3D5X9 | ISIN: CA64119M1059 | Ticker symbol: AIAI) develops solutions for the pharmaceutical industry to utilize Generative Artificial Intelligence (Gen AI).
time to read: 7 minutes
|
Author:
André Will-Laudien
ISIN:
NETRAMARK HOLDINGS INC | CA64119M1059
Table of contents:

"[...] In Canada, there is $1.75 of debt for every dollar of disposable income - and that was true even before the pandemic. [...]" Karim Nanji, CEO, Marble Financial
Author
André Will-Laudien
Born in Munich, he first studied economics and graduated in business administration at the Ludwig-Maximilians-University in 1995. As he was involved with the stock market at a very early stage, he now has more than 30 years of experience in the capital markets.
Tag cloud
Shares cloud
Breakneck disruption
Imagine the world spinning at the speed of light – that is exactly what is happening now wherever artificial intelligence comes into play. Business models that were thriving just yesterday are already outdated today, being swiftly replaced by new, smarter approaches. Data is being processed in record time as if centuries-old knowledge were available at the push of a button. Especially in the pharmaceutical industry, AI is now really taking off: it makes clinical studies more efficient and precise by sifting through an incredible amount of medical data, uncovering patterns, and thus improving decision-making. It is almost like a revolution – quite honestly, this changes everything! The Canadian tech company NetraMark Holdings Inc. (WKN: A3D5X9 | ISIN: CA64119M1059 | Ticker: AIAI) is developing groundbreaking solutions for the pharmaceutical world with generative AI. The results so far are an absolute quantum leap, and while NetraMark's market value is still in its early stages, time is of the essence - things are getting really exciting!
Recruitment and data analysis for clinical studies
In today's pharmaceutical world, AI has made a huge difference, especially when it comes to clinical studies. In the past, gathering and evaluating all patient data, laboratory results, and clinical reports was a real challenge. Today, AI can do this in just a few seconds, as if working at the speed of light. Automated pattern recognition uncovers connections that human analysts would often overlook. Deep learning algorithms help identify side effects and therapeutic successes early on. And the best part: AI can comb through electronic patient records and quickly find suitable study participants. This significantly shortens the often lengthy recruitment phase – a real game changer if you ask us!
Blockbuster Potential: NetraMark with NetraAI 2.0
NetraMark Holdings Inc. (WKN: A3D5X9 | ISIN: CA64119M1059 | Ticker: AIAI) is no longer just a small start-up – the Company is about to shake up the pharmaceutical market! In February, the Company introduced the latest version of its AI platform, NetraAI 2.0. This platform is the next generation to truly shake up clinical trials: it helps sponsors gain key insights, refine endpoints, and optimize the criteria for study initiation. In doing so, it lays the foundation for successful approvals. The pharmaceutical industry is already utilizing various AI models, and recent studies show that the market for AI in clinical research is set to explode – from around USD 1.3 billion in 2021 to an estimated USD 5.6 billion in 2029. That is a strong growth rate of almost 20% per year!
According to Mordorintelligence.com, in the pharmaceutical industry specifically, the market size for AI is expected to grow from an estimated USD 3.1 billion in 2024 to approximately USD 17.9 billion by 2029. Here, a compound annual growth rate (CAGR) of 43% can even be achieved. These figures illustrate AI's significant potential and growing importance in clinical research and healthcare. AI also helps to conduct virtual clinical trials, dramatically reducing costs and ethical challenges.
A New Era in Clinical Trial Optimization
NetraAI 2.0 takes a decisive step forward, solving one of the biggest challenges in clinical research: Finding the perfect balance between efficacy and feasibility. The platform transforms clinical trial data into clear, actionable insights, not only shortening study times but also improving decision-making for all stakeholders. AI-generated reports prioritize the most critical findings and help to adapt study strategies continuously. Using multiple validation layers, it identifies robust models that predict which patient groups are most likely to respond best to a therapy – and highlight potential risks. Unlike other AI methods that shoehorn every patient into one category, NetraAI separates the data into explainable and less explainable groups, ensuring that valuable details are not lost. Other AI methods lack these focusing mechanisms, assigning every patient to a class, even when it leads to "overfitting," drowning out important information that could have been used to improve the chances of a study's success. It is almost like magic – or rather, science that makes the difference.
Ontario Brain Institute collaboration on Imaging Research
Another milestone: NetraMark has partnered with the Ontario Brain Institute (OBI) to improve the analysis of imaging and multimodal data in severe depressive disorders. This collaboration will help to better distinguish and optimize treatment success in future clinical trials. The goal is to develop innovative neuroanalytics tools that efficiently process MRS, fMRI, and MEG data, taking machine learning to a new level. By utilizing graph-theoretical approaches, the initiative aims to improve our understanding of how patients respond to established and new therapies – a real step forward that will advance personalized medicine. The initiative will introduce graph-theoretic variables into a structured pipeline, improving understanding of how patients respond to both established and new treatments, such as escitalopram and ketamine.
"This collaboration with OBI is a significant milestone for NetraMark, enabling us to leverage AI to improve research in the field of mental health and clinical decision-making," said Joseph Geraci, Chief Scientific and Technical Officer of NetraMark. "By developing sophisticated machine learning pipelines, we are helping to advance personalized medicine, accelerate the discovery of neuropsychiatric disorders, and help our clients better understand their psychiatric clinical trials." This collaboration aligns with NetraMark's broader vision of using AI to gain deeper insights into complex diseases, with applications extending beyond MDD to other neuropsychiatric and neurological disorders. The partnership aims to underscore the potential of AI in improving precision medicine, optimizing clinical trial enrichment, and enhancing patient outcomes.
Strategic partnership in focus
NetraMark has also entered into a pilot collaboration agreement with a Top-5 pharmaceutical company. The goal is to use NetraAI technology to gain deeper insights into patient data and support the development of new treatments, particularly for autoimmune disorders. The Company's financial position was strengthened in the first quarter of this fiscal year, with warrants and stock options exercised, resulting in net proceeds of over CAD 1.16 million. This financing will enable NetraMark to expand operations and drive innovation in precision medicine – a clear advantage that should increase its market value over the long term.
Co-founder and CTO Dr. Joseph Geraci defines the target fields for the near future as follows: "From the outset, NetraAI was developed as a hub to enhance the ability of machine intelligence to understand patient subpopulations in clinical trials. As AI continues to advance at an unprecedented rate, NetraAI 2.0 puts us in a unique position to push the boundaries of innovation and redefine the way clinical trials are designed and understood."
The International Society for CNS Clinical Trials and Methodology (ISCTM) Conference Update
NetraMark presented two significant studies at the ISCTM conference demonstrating the power of advanced machine learning in clinical trials for major depressive disorder (MDD) and schizophrenia.
The first presentation showed that NetraAI-driven analysis of patient subpopulations resulted in a 28% increase in model accuracy compared to conventional ML approaches. Sensitivity improved by 31%, while specificity increased by 51%, reducing the rate of false positives. NetraAI identifies and explains the key variable combinations, providing deeper insights into drug and placebo responses. Performance improved significantly when NetraAI-derived variables were included in traditional ML methods.
The second presentation from NetraMark was dedicated to the discovery of predictive biomarkers in schizophrenia using advanced machine learning to decode heterogeneity. It demonstrated the ability of NetraAI to learn from heterogeneous patient populations in schizophrenia studies. Using data from the CATIE schizophrenia trial, NetraAI identified clinically meaningful subpopulations that preferentially respond to olanzapine or perphenazine.
NetraMark's AI-driven methods have the potential to transform the landscape of clinical CNS research by improving patient stratification for more targeted studies, reducing placebo response and study failure, and accelerating drug development by improving predictive modeling.
Conclusion: The potential is gigantic
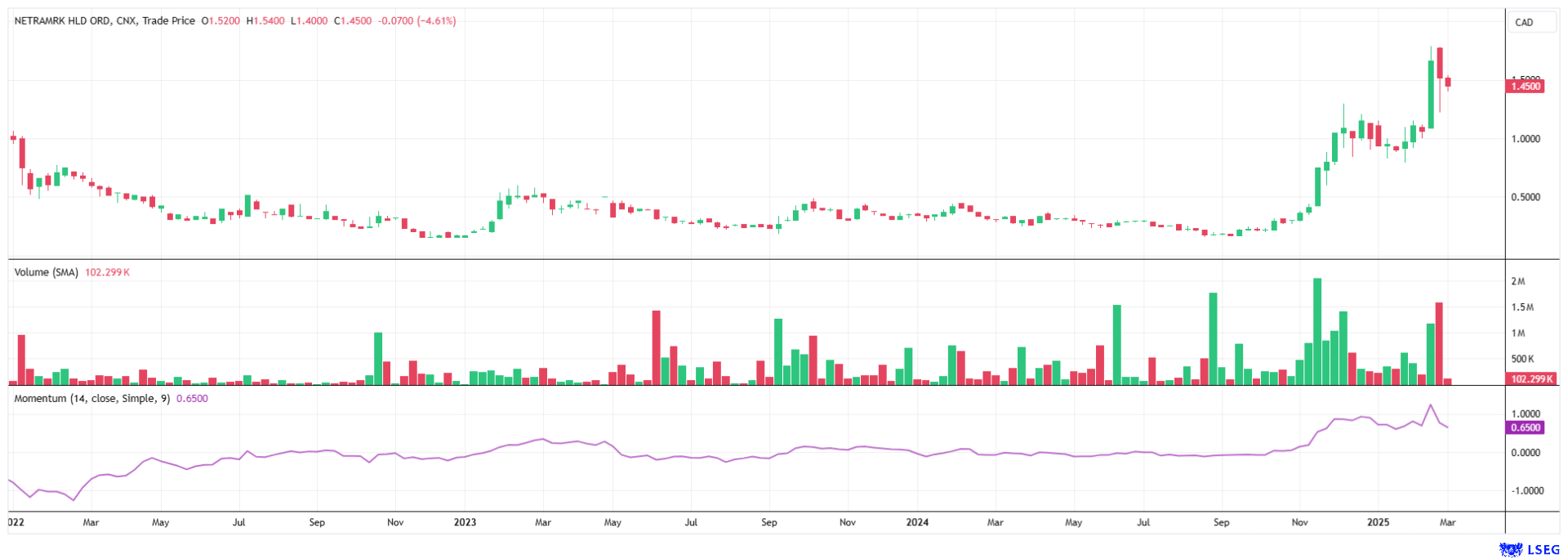
The NetraMarkshare (WKN: A3D5X9 | ISIN: CA64119M1059 | Ticker symbol: AIAI) experienced a strong upward trajectory after a quiet 2024, beginning in October. Coinciding with the development of the latest AI solution, NetraAI, the stock price surged from around CAD 0.20 to a peak of CAD 1.55. In the first quarter, the Company stands to benefit from the positive sentiment in the high-tech sector. With the rollout beginning and the current collaboration with a major pharmaceutical company, the visibility of NetraMark's solutions should increase and lead to new customers and revenue. The business model should gain significant momentum very quickly with further collaborations.
Conclusion: The current price of CAD 1.30 should be seen only as a temporary stop on the way to a fair valuation. From a technical chart perspective, the price broke out in November 2024 with high volume, and since then, the new price level has stabilized significantly. For investors in Germany, the stock is also tradable in Frankfurt and Munich. In light of NetraAI's broad range of applications, a large field of activity within the biotech and pharmaceutical sectors, and excellent peer group, we expect a rapid revaluation based on the current market capitalization of CAD 100 million.
Further advances in AI-powered analysis could lead to personalized drug trials that develop tailored therapeutic approaches for individual patient groups. In addition, AI could make the entire approval process more transparent and efficient. NetraMark Holdings is on the verge of becoming one of the first major AI innovators in the area of clinical development. However, the Company's market capitalization is only EUR 66 million. Overall, NetraMark is a promising player that – despite short-term volatility – could deliver significant results in the long term. Time is of the essence!
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") currently hold or hold shares or other financial instruments of the aforementioned companies and speculate on their price developments. In this respect, they intend to sell or acquire shares or other financial instruments of the companies (hereinafter each referred to as a "Transaction"). Transactions may thereby influence the respective price of the shares or other financial instruments of the Company.
In this respect, there is a concrete conflict of interest in the reporting on the companies.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is also a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.