September 29th, 2025 | 07:00 CEST
Takeover in the Ozempic market! BioNxt Solutions next? Novo Nordisk, Eli Lilly, and Pfizer are fighting!
The battle against obesity is considered one of the biggest growth markets in the global pharmaceutical sector. According to estimates, the market for obesity therapies could reach a volume of up to USD 150 billion by 2030. So far, Novo Nordisk and Eli Lilly have dominated the market. But almost every major corporation and innovative challenger wants a piece of the pie. The latest example: Pfizer is planning a billion-dollar takeover of US-based Metsera, a company competing with novel drugs against obesity. BioNxt Solutions is also a hot takeover candidate. The Company aims to revolutionize the market and attract new customers with an oral dosage form, and the first prototypes have already been developed. That makes it an attractive prospect for virtually every pharmaceutical company.
time to read: 4 minutes
|
Author:
Fabian Lorenz
ISIN:
Bionxt Solutions Inc. | CA0909741062 , NOVO NORDISK A/S | DK0062498333 , ELI LILLY | US5324571083 , PFIZER INC. DL-_05 | US7170811035
Table of contents:

"[...] As a company dedicated to developing treatments for rare heart diseases, we see this as an opportune moment to contribute to the fight against heart disease and make meaningful strides in improving heart health worldwide. [...]" David Elsley, CEO, Cardiol Therapeutics Inc.
Author
Fabian Lorenz
For more than twenty years, the Cologne native has been intensively involved with the stock market, both professionally and privately. He is particularly passionate about national and international small and micro caps.
Tag cloud
Shares cloud
Competition for the best technologies is underway
According to analyst estimates, the market for GLP-1-based obesity therapies such as Ozempic (Novo Nordisk) and Mounjaro (Eli Lilly) is expected to reach a volume of around USD 150 billion by the end of the decade. It is therefore not surprising that numerous companies, such as Roche and Amgen, are working on their own preparations. Most recently, the Financial Times reported that Pfizer intends to acquire the biotech company Metsera. The US pharmaceutical giant aims to secure early access to a potential blockbuster with the acquisition. Metsera is working on innovative active ingredients for obesity, which are still in clinical development. This could help Pfizer make up ground in the race against market leaders Novo Nordisk and Eli Lilly after failing with its own development. Pfizer is offering a 40% premium per Metsera share and intends to pay a total of USD 3.5 billion. After missing out on the GLP-1 boom with its own projects, the transaction shows that pharmaceutical companies are willing to dig deep into their pockets to keep up. For investors, one thing is clear: the competition for the best technologies in the obesity market is underway.
Acquisition candidate BioNxt Solutions
Against this backdrop, the Canadian-German bioscience company BioNxt Solutions is also coming into sharper focus. The Company is working on an innovative technology that has the potential to revolutionize the treatment of overweight and obesity. At its heart is an oral dissolvable film in which active ingredients such as semaglutide (Ozempic) can be embedded—a drug that has previously only been available in the form of injections. The idea is that patients will in future be able to simply let a small film melt in their mouths instead of having to give themselves regular injections. This would not only increase patient acceptance and adherence to therapy but also create a clear competitive advantage for BioNxt. This is because ease of use plays a decisive role, especially in the mass market for obesity therapies. BioNxt is initially focusing on market leader Ozempic. The Danish company generated sales of around USD 12 billion in the last quarter alone. However, the technology is likely to be transferable to all active ingredients. This makes BioNxt an interesting takeover candidate for any company in the field.
Semaglutide dissolvable film prototypes already developed
Proof-of-concept studies for the development of the orally soluble film with semaglutide were recently successfully completed. The prototypes with strong performance characteristics demonstrated rapid disintegration, homogeneous film quality, good processability during manufacture, and favorable mechanical properties. A patent application for the semaglutide dissolvable film is expected to be filed in the coming months. BioNxt is also working on further refining the formulation, with a clinical pilot study scheduled to begin as early as next year.
Technology already proven
What is more, BioNxt is not starting from scratch. The Company already has regulatory-validated platform technologies in the field of oral drug delivery that have been tested for other indications. This foundation facilitates the approval of additional products and reduces development risks – a point that makes the Company particularly interesting for potential partners or buyers. With a market capitalization of less than CAD 100 million, BioNxt currently appears to be a "hidden champion." For big pharma, this could be the perfect time to secure access to a disruptive technology before the Company's value skyrockets with market launch.
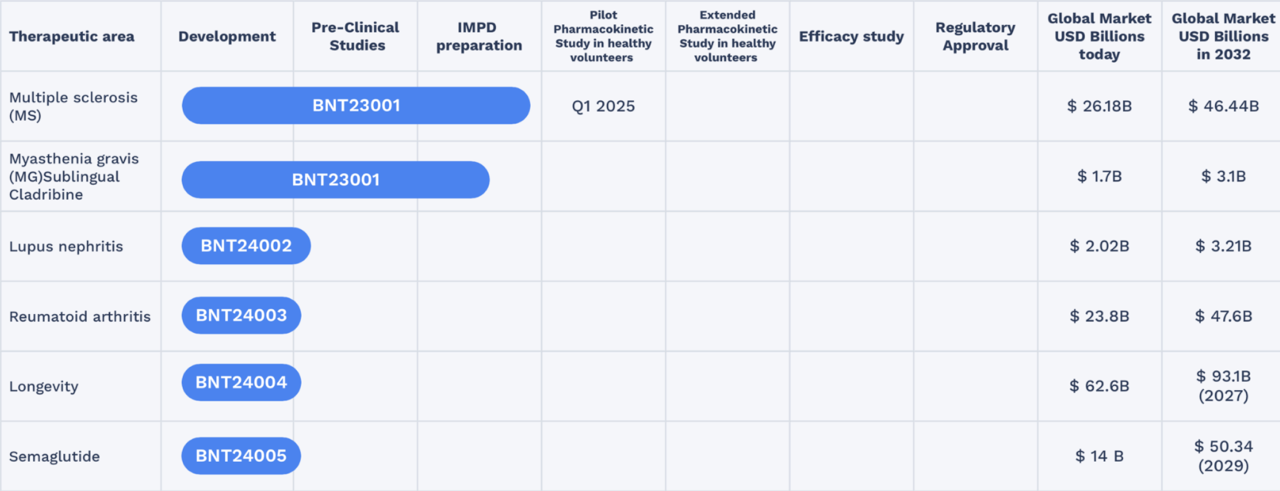
Flagship project targets multiple sclerosis blockbuster
The Ozempic project is not the only one with which BioNxt is targeting a billion-dollar market. The Company is working intensively on its flagship project BNT23001, an orally dissolvable film formulation of the drug cladribine for the treatment of multiple sclerosis. This dosage form is designed to reduce the typical side effects associated with tablets or injectable MS drugs. For patients, this means greater comfort and less burden—factors that can be crucial in the long-term treatment of chronic diseases. BioNxt has already secured key patents for BNT23001, covering not only the transdermal delivery technology but also the specific formulation. In the US, BioNxt has filed its patent application for BNT23001 under the accelerated "Track One" procedure. Success would have a very positive impact on clinical development and subsequent commercialization. This means that the project is well secured both scientifically and commercially. Given a global MS market of over USD 25 billion, this opens up an opportunity for BioNxt to occupy a niche that is both medically urgent and economically highly attractive.
Important date: On October 8, 2025, BioNxt Solutions will present at the virtual 16th International Investment Forum (IIF). Private and institutional investors can register here to participate free of charge. Many exciting German and international small and mid-caps will be presenting.
Conclusion: Market capitalization with significant upside potential
BioNxt Solutions is working on innovative forms of administration. Investors currently have an exciting opportunity to get in on the action. If the Company continues to be so successful in its development, there can really only be two possibilities: either the share price will rise significantly or Big Pharma will make a takeover bid. Both are attractive scenarios.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may hold shares or other financial instruments of the aforementioned companies in the future or may bet on rising or falling prices and thus a conflict of interest may arise in the future. The Relevant Persons reserve the right to buy or sell shares or other financial instruments of the Company at any time (hereinafter each a "Transaction"). Transactions may, under certain circumstances, influence the respective price of the shares or other financial instruments of the Company.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.




