September 11th, 2025 | 07:10 CEST
Forget weight loss injections! BioNxt Solutions is unsettling Big Pharma, but could become an important partner
There is a catch with weight loss injections: they are injections. Studies show that adherence to treatment drops dramatically after one year. What if the blockbuster drug could simply be dissolved under the tongue? That is precisely what BioNxt Solutions is working on. With its patented dissolvable film technology, the Canadian-German biotech company not only aims to revolutionize obesity therapy but also make strides in multiple sclerosis treatment. The approach could transform entire markets.
time to read: 4 minutes
|
Author:
Armin Schulz
ISIN:
Bionxt Solutions Inc. | CA0909741062
Table of contents:

"[...] Defence will continue to develop its Antibody Drug Conjugates "ADC" and its radiopharmaceuticals programs, which are currently two of the hottest products in demand in the pharma industries where significant consolidations and take-overs occurred. [...]" Sébastien Plouffe, CEO, Founder and Director, Defence Therapeutics Inc.
Author
Armin Schulz
Born in Mönchengladbach, he studied business administration in the Netherlands. In the course of his studies he came into contact with the stock exchange for the first time. He has more than 25 years of experience in stock market business.
Tag cloud
Shares cloud
One platform, many active ingredients: The technology behind the vision
Imagine if you could simply place your medication on your tongue like a piece of sugar. It dissolves within seconds, and the active ingredient is absorbed directly into the bloodstream via the oral mucosa without passing through the stomach and liver. BioNxt Solutions is pursuing this vision with its oral dissolvable film (ODF) platform. For investors, it is the vision of a technological leap that could turn a small biotech company into a sought-after partner for the pharmaceutical giants.
The Company has not limited itself to a single active ingredient. Instead, it acts as a specialized developer of delivery systems. Its innovation is the platform itself. "We have built a great platform over the past 12 months, both in terms of capital markets and our future product candidates. With three distinct and proprietary programs for the development of sublingual drugs currently underway in Europe and a promising contract for the delivery of chemotherapeutic drugs nearing completion here in North America, the Company is well positioned for value growth over the next 12 months," confirms CEO Hugh Rogers. This flexibility opens up several opportunities.
Cladribine for MS: Proof of concept within reach
The most prominent approach concerns the active ingredient cladribine for the treatment of multiple sclerosis (MS). The market for MS therapies is huge and growing steadily. The issue is that many patients suffer from swallowing difficulties, which makes it difficult for them to take conventional tablets. BioNxt elegantly circumvents this problem. Its sublingual cladribine film, called BNT23001, requires no water and no swallowing. The efficacy of the active ingredient is already known, which significantly shortens the development path. The decisive factor is proof of bioequivalence - the film works just as well as the tablet. If this is successful, there would be little standing in the way of approval.
The strategic protection of this technology is already well advanced. The core patents have already been accepted in Europe and Eurasia, and a fast-track procedure is underway in the US, which could lead to a decision by spring 2026. It is important to note that patent protection applies not only to cladribine but also to the application of the platform for an entire class of neurological indications. This creates a competitive advantage that extends far beyond a single product.
The partnership with Gen-Plus: Expertise from Munich
The importance of the German partner Gen-Plus cannot be overstated. Gen-Plus provides GMP-compliant laboratory infrastructure and contributes crucial expertise in the fields of polymer science and peptide formulation. This partnership enables BioNxt to pursue a rapid and regulatory-compliant development process aligned with European standards - all without the need for heavy capital investments in infrastructure. It is an asset-light approach that helps conserve the Company's capital.
Semaglutide delivery: Entry into the billion-dollar GLP-1 market
The biggest buzz right now is the entry into the ultimate hype market: GLP-1 receptor agonists like semaglutide, known as Ozempic or Wegovy. There has recently been a decisive momentum in development: On September 2, BioNxt announced that it had received the high-purity active ingredient semaglutide. This delivery marks the start of practical laboratory work.
"Now that we have the active ingredient available on site, we can put theory into practice," said CEO Rogers. Preformulations and tests are now underway in the laboratories of German partner company Gen-Plus, a licensed developer of pharmaceutical thin-film formulations. The team is characterizing the stability of the peptide and working on optimizing the film matrix. The specific goal is to file a provisional patent application in the third quarter of 2025.
The market for these active ingredients is estimated at over USD 150 billion by 2030. The key problem here is also the application. Most preparations have to be injected. According to figures, treatment adherence drops to around 32% after just one year. A non-invasive alternative is the "Holy Grail." The logic behind BioNxt's approach is compelling: The Company does not want to replace the active ingredient, but instead radically improve its dosage form.
OTCQB listing: Greater visibility for US investors
In parallel with its technical development, BioNxt is also optimizing its capital market presence. Since September 5, the Company's shares have been listed not only on the open OTC Pink Market, but also on the higher-quality OTCQB Market in the US under the symbol "BNXTF." This uplisting is more than just a symbolic act.
It subjects the Company to stricter transparency and reporting requirements, making it investable for a broader, more institutional audience in the US. Increased attention and improved liquidity could support the share price in the long term. Analysts at Black Research have given the stock a "Strong Buy" rating and see a price target of EUR 2.50. The stock is currently trading at EUR 0.602, which corresponds to approximately CAD 0.94.
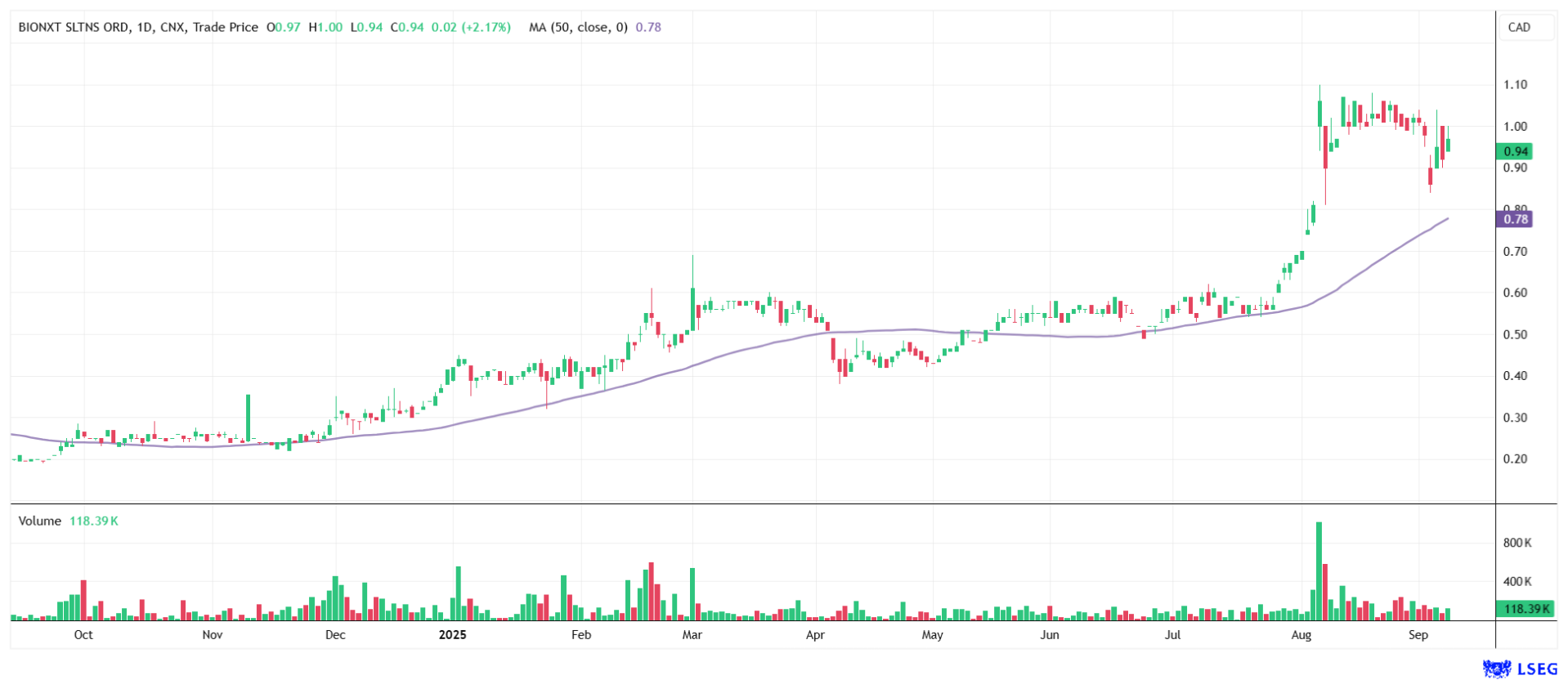
BioNxt Solutions is not about inventing the next blockbuster drug - it is focused on delivering existing ones better. Recent developments reinforce this strategy. The delivery of semaglutide is driving the highly anticipated project forward, while the OTCQB listing is increasing its reach among investors. The Company's asset-light approach is strategically clever - it reduces development risks and costs while positioning BioNxt as an attractive partner or takeover target for large pharmaceutical companies. For risk-aware investors, this presents an opportunity to get in early on a platform that has the potential to transform how treatments are delivered.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may hold shares or other financial instruments of the aforementioned companies in the future or may bet on rising or falling prices and thus a conflict of interest may arise in the future. The Relevant Persons reserve the right to buy or sell shares or other financial instruments of the Company at any time (hereinafter each a "Transaction"). Transactions may, under certain circumstances, influence the respective price of the shares or other financial instruments of the Company.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.




