September 16th, 2025 | 07:00 CEST
Hot Stock shakes up Big Pharma! BioNxt Solutions aims to replace Ozempic weight loss injection!
BioNxt is currently shaking up Big Pharma. The announcement that it plans to replace the Ozempic weight loss injection with an oral dissolvable film is once again fueling speculation about the share price. Just like in the multiple sclerosis space, the Company aims to disrupt a billion-dollar obesity market. Further exciting operational news is expected in the coming months. Key patents are already in place. With a market capitalization of around CAD 100 million, the Canadian-German life sciences company is far from expensive – even for Big Pharma. The major corporations will undoubtedly be watching BioNxt's development very closely in the coming months. Until then, the stock is likely to trade significantly higher.
time to read: 3 minutes
|
Author:
Fabian Lorenz
ISIN:
Bionxt Solutions Inc. | CA0909741062
Table of contents:

"[...] As a company dedicated to developing treatments for rare heart diseases, we see this as an opportune moment to contribute to the fight against heart disease and make meaningful strides in improving heart health worldwide. [...]" David Elsley, CEO, Cardiol Therapeutics Inc.
Author
Fabian Lorenz
For more than twenty years, the Cologne native has been intensively involved with the stock market, both professionally and privately. He is particularly passionate about national and international small and micro caps.
Tag cloud
Shares cloud
Stock poised for next price jump
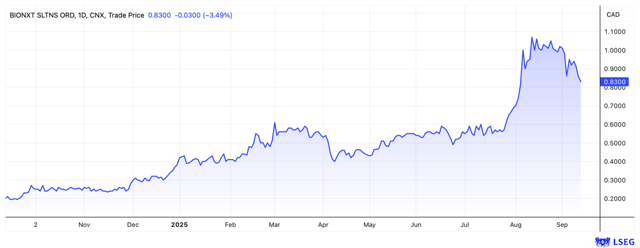
BioNxt's stock has impressively demonstrated its strength in recent weeks. Starting at EUR 0.35 at the end of July, the share launched a spectacular rally, climbing to EUR 0.66 in a short time before undergoing a healthy consolidation to its current level of EUR 0.53. From both a technical and fundamental perspective, the period of calm could now be over, as the conditions for the next price surge are in place.
Innovations for new drug delivery formats
What makes this German-Canadian company so exciting? BioNxt is developing modern drug delivery systems designed to make medications more accessible to patients while also improving their effectiveness. In addition to transdermal patches and traditional tablets, the Company focuses particularly on oral dissolvable films that dissolve directly in the mouth, enabling rapid absorption. The concept of releasing chemotherapeutic agents directly at the tumor site further underscores its patient-centered approach. The goal is to reduce side effects and increase treatment adherence—a crucial factor in managing chronic diseases. With this strategy and consistent protection through international patents, BioNxt is positioning itself in high-growth markets.
Melting Ozempic instead of injecting it
BioNxt Solutions' latest project is working on a dissolvable film formulation of semaglutide. The active ingredient is at the heart of Novo Nordisk's blockbuster drugs Ozempic and Wegovy for obesity and diabetes, which generate billions in revenue – and the trend is still rising. BioNxt's goal is to develop an oral dosage form that absorbs the active ingredient through the oral mucosa, thereby replacing injections. If this step is successful, a patent could soon follow – a milestone that would make the Company an attractive target for partnerships with industry giants or even acquisitions. The timing is favorable: Novo Nordisk and Eli Lilly currently dominate the market, but demand significantly exceeds production capacity. The market for obesity drugs is far from saturated. According to analysts, the industry could generate around USD 150 billion in revenue by the end of the decade. Nearly all medications must be injected - a barrier for many patients. An easy-to-take, effective orally disintegrating film variant could therefore significantly increase willingness to undergo treatment and position BioNxt as an innovative player in one of the most lucrative pharmaceutical segments.
Several milestones achieved recently
But BioNxt has even more exciting projects in the pipeline. Several milestones were achieved over the summer. At the end of July, a prototype of the lead candidate BNT23001 was presented – a cladribine dissolvable film formulation for multiple sclerosis. Initial tests confirmed consistent quality, rapid disintegration times and stable drug loading. This could make a decisive difference, especially for MS patients, who often suffer from swallowing disorders. Shortly thereafter, BioNxt reported significant progress in the patent field: Both the European Patent Office and the Eurasian Organization confirmed key property rights for the technology. At the same time, an accelerated procedure is underway in the US, which could lead to a patent being granted within a year. This is flanked by a platform patent that secures the dissolvable film technology far beyond cladribine. Looking ahead to the coming months, management anticipates preclinical data and further partnerships that will strengthen the foundation for clinical development and market entry.
Conclusion: New share price rally and takeover speculation
With its innovative dissolvable film technologies, BioNxt Solutions has positioned itself in a way that makes the Company exciting for investors and potential partners alike. While the development of an oral version of Ozempic is making headlines, the progress in MS therapy with cladribine and the secure patent strategy show that BioNxt has a solid foundation. With a comparatively low valuation and several projects in growth markets, the stock could benefit from further milestones in the near future – and Big Pharma is likely to be watching very closely.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may hold shares or other financial instruments of the aforementioned companies in the future or may bet on rising or falling prices and thus a conflict of interest may arise in the future. The Relevant Persons reserve the right to buy or sell shares or other financial instruments of the Company at any time (hereinafter each a "Transaction"). Transactions may, under certain circumstances, influence the respective price of the shares or other financial instruments of the Company.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.