October 30th, 2025 | 07:15 CET
BioNxt Solutions: The underrated biotech gem you need to know - Potential partner for Merck and Novo Nordisk
Investing in biotech often feels like a rollercoaster ride. The sector regularly delivers groundbreaking success stories, yet just as often, promising studies come to nothing. For investors, this usually means a nerve-wracking journey with long dry spells and a true all-or-nothing gamble. But what if there was a way to avoid the typical risks without sacrificing the enormous upside potential? The Canadian company BioNxt Solutions is pursuing precisely this approach and could very well change the rules of the game in the biotech industry.
time to read: 4 minutes
|
Author:
Armin Schulz
ISIN:
Bionxt Solutions Inc. | CA0909741062
Table of contents:

"[...] Defence will continue to develop its Antibody Drug Conjugates "ADC" and its radiopharmaceuticals programs, which are currently two of the hottest products in demand in the pharma industries where significant consolidations and take-overs occurred. [...]" Sébastien Plouffe, CEO, Founder and Director, Defence Therapeutics Inc.
Author
Armin Schulz
Born in Mönchengladbach, he studied business administration in the Netherlands. In the course of his studies he came into contact with the stock exchange for the first time. He has more than 25 years of experience in stock market business.
Tag cloud
Shares cloud
The smart shortcut: Proven active ingredients, repackaged
BioNxt Solutions is not reinventing the wheel; it is simply making it better. Instead of betting on unproven new active ingredients, the Company reformulates already approved and well-established drugs. Using a proprietary platform technology, BioNxt transforms these into orally soluble, sublingual thin-films that dissolve under the tongue.
This approach brings significant advantages. The basic safety and efficacy of the drugs are already proven. Instead of lengthy and costly Phase 2 or 3 studies, development focuses on demonstrating bioequivalence. This significantly shortens the path to market readiness.
"We can develop a reformulated, patented drug in sublingual form in maybe two to four years and for just a few million USD," says CEO Hugh Rogers. For a small publicly traded company, this represents a calculable and financeable risk.
The current animal study: The foundation for the next big step
BioNxt is now proving that its model is more than just theory. The upcoming animal study for the lead product BNT23001 marks a decisive step in preclinical validation. This study will provide the essential data for dosing in the planned human studies.
"The Company has already confirmed bioequivalence in low body weight animals (<20 kg). However, only an animal model with a higher body weight (>40 kg) will provide valuable insights into the appropriate sublingual dose amount for humans," explains Hugh Rogers of BioNxt.
The timeline is concrete. The study is expected to be completed in November, with results anticipated in December. This is the final preclinical step before the comparative bioequivalence study in humans, which is planned for early 2026.
The pipeline: Focus on billion-dollar markets
BioNxt focuses its resources on indications with established markets and clearly defined needs. The flagship product is BNT23001, an oral dissolvable film formulation of cladribine for the treatment of multiple sclerosis (MS).
Cladribine is the active ingredient behind Merck's MS therapy Mavenclad. The reformulation as a film addresses a specific problem: about 40% of MS patients suffer from swallowing difficulties. An orally soluble film could significantly improve treatment adherence.
But BioNxt is thinking ahead. A second promising project is the development of an oral dissolvable film for semaglutide, the active ingredient behind bestsellers like Ozempic and Wegovy. The market for GLP-1 receptor agonists is booming, with sales of over USD 29 billion for semaglutide alone in 2024. If the injection is no longer necessary, even more people could turn to this weight loss drug.
In addition to the MS drug and the semaglutide project, the Company is working on a novel platform for targeted chemotherapy. In vitro data indicate up to a tenfold increase in therapeutic efficacy with improved safety.
The business model: Licensing as an exit strategy
BioNxt takes a different approach than most pharmaceutical companies. Instead of building its own sales network, the Company deliberately focuses on its true strengths: research and development, protecting inventions, and preparing clinical trials.
The products will later be marketed through licenses or direct sales to the major industry players. Merck, a market leader, has already occupied the market for cladribine. A newly formulated version of the active ingredient that provides real benefits for patients could therefore be an extremely attractive asset for acquisition or licensing.
This business model has a clear advantage: BioNxt can focus on what it truly does best while avoiding the enormous costs of sales and marketing. This is also attractive for investors, as advance payments and milestone payments often provide revenue much earlier.
Patent protection: The key to competitive success
For platform technologies, the strength of intellectual property determines success. BioNxt is systematically building a robust protective shield in this area. The first so-called "umbrella patent" for sublingual administration is already in the national phase in the US, Europe, Canada, Japan, Australia, and other key markets.
A real game-changer is the accelerated examination by the US Patent Office under the Track One program. Instead of being tied up in years of proceedings, BioNxt expects a decision here within just 9 to 12 months. This strategic step strengthens the negotiating position in the run-up to the decisive bioequivalence studies.
The portfolio is being actively expanded. Additional patent families are to be filed in the coming weeks and months, making it increasingly difficult for competitors to enter the space.
Cost-effectiveness: Small amounts, big impact
What makes the model particularly interesting for investors is its efficient use of capital. "For USD 250,000, we can conduct a human study over several weeks," emphasizes the CEO. These comparatively low costs mean more frequent news cycles and less capital tied up.
While the development of a new active ingredient can easily cost hundreds of millions of USD, BioNxt operates on a completely different scale. The Company can achieve substantial progress and milestones with comparatively modest resources.
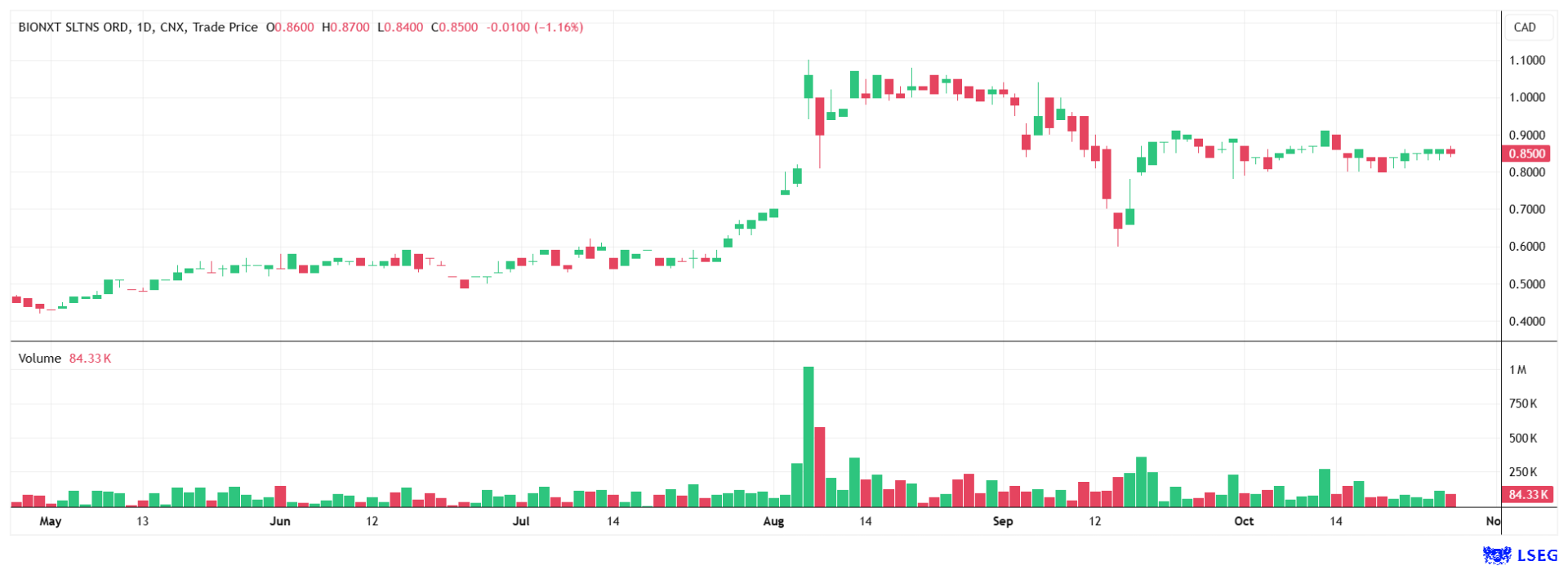
The stock is currently trading at CAD 0.85, significantly below Black Research's price target of EUR 2.50, which is roughly equivalent to CAD 4.05.
BioNxt Solutions takes a refreshingly sober approach in the often hype-driven biotech sector. By focusing on reformulating existing bestsellers with its patented platform technology, the Company significantly reduces the typical risks and time horizons. The upcoming animal study underscores this systematic approach and forms a solid foundation for the human studies planned for 2026. For investors looking for a biotech investment with a calculable risk profile and substantial upside potential, BioNxt Solutions offers a compelling alternative.
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") may hold shares or other financial instruments of the aforementioned companies in the future or may bet on rising or falling prices and thus a conflict of interest may arise in the future. The Relevant Persons reserve the right to buy or sell shares or other financial instruments of the Company at any time (hereinafter each a "Transaction"). Transactions may, under certain circumstances, influence the respective price of the shares or other financial instruments of the Company.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.