October 24th, 2025 | 07:35 CEST
BioNxt Solutions advances sublingual MS therapy into decisive development phase
BioNxt Solutions is on the verge of a decisive step in the development of its novel sublingual cladribine formulation for the treatment of multiple sclerosis. Following successful small-animal studies, the Company is now launching a large-scale animal study to optimize dosage ahead of the planned human bioequivalence study in 2026. The orally soluble thin-film technology is designed to enable faster drug absorption, greater bioavailability, and easier administration. At the same time, BioNxt is securing patent protection in key global markets, further strengthening its position in the competitive field of advanced drug delivery systems. With its focus on patient-friendly, precise, and effective therapies, BioNxt could set a new standard in MS treatment. A promising setup for the coming months. What opportunities does the Company offer dynamic investors?
time to read: 4 minutes
|
Author:
André Will-Laudien
ISIN:
Bionxt Solutions Inc. | CA0909741062
Table of contents:

"[...] Defence will continue to develop its Antibody Drug Conjugates "ADC" and its radiopharmaceuticals programs, which are currently two of the hottest products in demand in the pharma industries where significant consolidations and take-overs occurred. [...]" Sébastien Plouffe, CEO, Founder and Director, Defence Therapeutics Inc.
Author
André Will-Laudien
Born in Munich, he first studied economics and graduated in business administration at the Ludwig-Maximilians-University in 1995. As he was involved with the stock market at a very early stage, he now has more than 30 years of experience in the capital markets.
Tag cloud
Shares cloud
New approaches to drug delivery
BioNxt Solutions, a German-Canadian biotech company with research facilities in North America and Europe, develops platform technologies that aim to deliver existing drugs more effectively, safely, and in a patient-friendly manner. The Company focuses on three key areas: sublingual thin-film formulations, transdermal patches, and targeted drug delivery for cancer therapies. Progress is being made across all fronts. These technologies aim to increase bioavailability, reduce dosages, and improve patient adherence. Such platforms have the potential to transform treatment landscapes, particularly for chronic diseases such as multiple sclerosis (MS), obesity, or MASH (fatty liver disease). With the development of patented systems for absorption via the oral mucosa or skin, BioNxt is addressing several billion-dollar markets that, according to expert studies, are projected to grow significantly in the coming years. One project is currently the focus of scientific and clinical interest: the novel sublingual cladribine formulation BNT23001 for MS therapy.
Preclinical progress with BNT23001 – Optimization prior to human trials
On October 21, 2025, BioNxt announced the start of a large-scale 15-day animal study to optimize the dosage of its lead product candidate, BNT23001. This study represents the final preclinical phase before the planned human bioequivalence study begins in 2026. BNT23001 is a sublingual, orally soluble thin-film formulation of cladribine, a proven immunomodulatory agent for the treatment of multiple sclerosis (MS). Administration via the oral mucosa is intended to enable faster drug absorption, higher bioavailability, and easier administration, particularly for patients with swallowing difficulties or who desire non-invasive alternatives to tablets or injections. Previous studies have already demonstrated bioequivalence in small-animal models. The study currently underway in large animals (over 40 kg) is intended to provide more precise data on dosage and pharmacokinetics, thereby laying the foundation for determining the optimal human dose. According to CEO Hugh Rogers, the study will provide insight into the optimal concentration per dose ("drug load per dose") and document the possible effects of above-average drug absorption ("super bioavailability"). The results are expected in December 2025.
Focus on cladribine: Potential to simplify MS therapy
Cladribine is considered an effective and well-established immunomodulator in MS treatment. However, the current tablet form requires precise dosing and is associated with potential side effects. A sublingual alternative such as BNT23001 could offer decisive advantages here:
• Faster onset of action through direct absorption via the oral mucosa
• Better bioavailability with lower drug amounts
• Higher treatment adherence due to easy administration without water or injections
In preclinical tests, BNT23001 demonstrated high absorption rates, bioequivalence to standard preparations, and no evidence of toxicity. This represents an important milestone for the transition to clinical trials in humans. With the upcoming human bioequivalence study planned for early 2026, BioNxt is entering a critical phase. The goal is to directly compare the new sublingual formulation with the approved cladribine tablet to confirm pharmacokinetic equivalence and potential therapeutic advantages.
Global patent strategy and regulatory positioning
In parallel with preclinical development, BioNxt is actively advancing the international protection of its technology. Patent nationalization is currently underway in key markets, including Europe, Canada, Australia, New Zealand, Japan, and Eurasia, as well as in the United States under a Track 1 priority procedure. Both the European Patent Office and the Eurasian Patent Office have already published positive notices of grant. This means that the essential criteria of novelty, inventive step, and industrial applicability have been officially recognized. This legal protection strengthens BioNxt's position ahead of the transition to clinical development and potential partnerships with larger pharmaceutical companies.
CEO Hugh Rogers explains the immense opportunities that pharmaceutical markets offer BioNxt on the International Investment Forum (IIF). The interview was conducted by Lyndsay Malchuk.
Outlook: Step by step toward clinical validation
With the launch of the dosing optimization study and preparations for the upcoming human bioequivalence study, BioNxt Solutions is positioning itself at a crucial interface between preclinical research and clinical validation. If the sublingual cladribine formulation demonstrates bioequivalence and clinical advantages, it could set a new benchmark for patient-friendly administration of MS therapies. In addition, BioNxt is working on other platforms, including transdermal patches and an innovative targeted delivery system for chemotherapy, designed to concentrate active ingredients directly in tumor tissue. This pipeline underscores the Company's ambition to fundamentally transform drug delivery, from drug release to the overall patient experience.
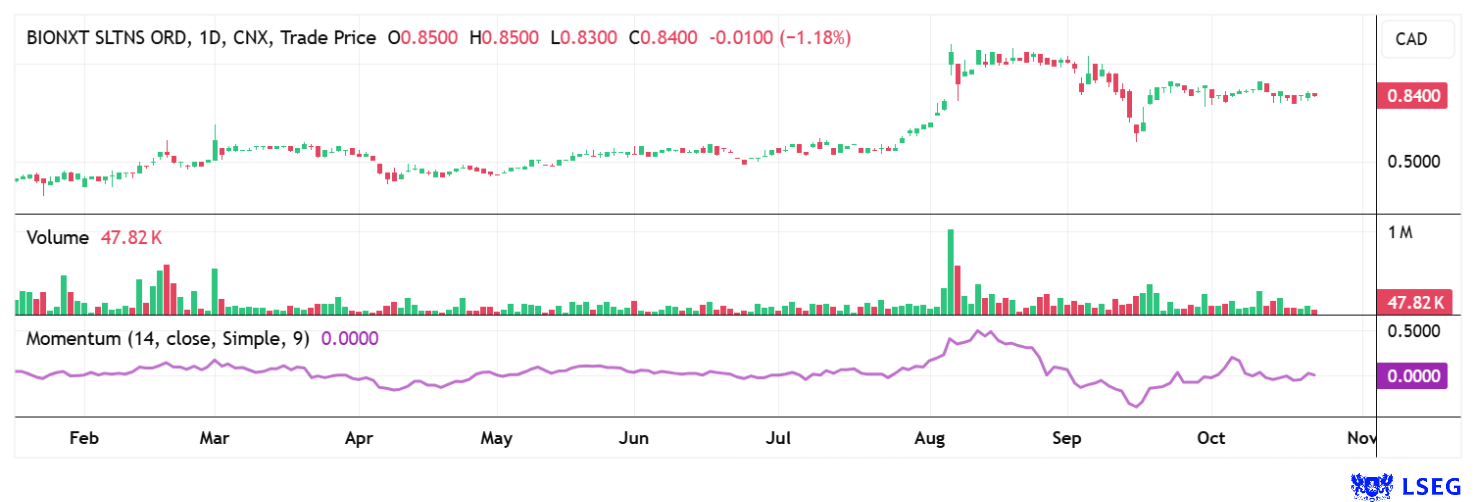
BioNxt Solutions is on the verge of an important milestone in the development of a novel sublingual MS therapy. The upcoming human bioequivalence study in 2026 will show whether the technology has the potential to change existing treatment paradigms. At the same time, the Company is strengthening its scientific and commercial base with its broad patent strategy and additional drug platforms. With the latest financing, the coffers are full for further steps through 2026. After a brief correction in October, the share price picked up speed again and climbed to CAD 0.85. This brings the 115.76 million shares to a market value of CAD 98.4 million - far too cheap for a biotech platform with this level of momentum!
Conflict of interest
Pursuant to §85 of the German Securities Trading Act (WpHG), we point out that Apaton Finance GmbH as well as partners, authors or employees of Apaton Finance GmbH (hereinafter referred to as "Relevant Persons") currently hold or hold shares or other financial instruments of the aforementioned companies and speculate on their price developments. In this respect, they intend to sell or acquire shares or other financial instruments of the companies (hereinafter each referred to as a "Transaction"). Transactions may thereby influence the respective price of the shares or other financial instruments of the Company.
In this respect, there is a concrete conflict of interest in the reporting on the companies.
In addition, Apaton Finance GmbH is active in the context of the preparation and publication of the reporting in paid contractual relationships.
For this reason, there is also a concrete conflict of interest.
The above information on existing conflicts of interest applies to all types and forms of publication used by Apaton Finance GmbH for publications on companies.
Risk notice
Apaton Finance GmbH offers editors, agencies and companies the opportunity to publish commentaries, interviews, summaries, news and the like on news.financial. These contents are exclusively for the information of the readers and do not represent any call to action or recommendations, neither explicitly nor implicitly they are to be understood as an assurance of possible price developments. The contents do not replace individual expert investment advice and do not constitute an offer to sell the discussed share(s) or other financial instruments, nor an invitation to buy or sell such.
The content is expressly not a financial analysis, but a journalistic or advertising text. Readers or users who make investment decisions or carry out transactions on the basis of the information provided here do so entirely at their own risk. No contractual relationship is established between Apaton Finance GmbH and its readers or the users of its offers, as our information only refers to the company and not to the investment decision of the reader or user.
The acquisition of financial instruments involves high risks, which can lead to the total loss of the invested capital. The information published by Apaton Finance GmbH and its authors is based on careful research. Nevertheless, no liability is assumed for financial losses or a content-related guarantee for the topicality, correctness, appropriateness and completeness of the content provided here. Please also note our Terms of use.




